Computational Analysis
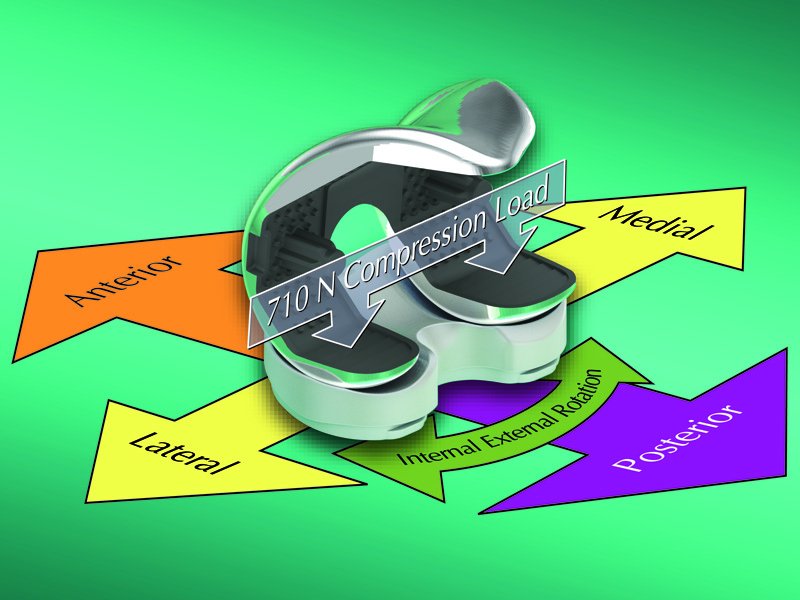
Clinical longevity of knee arthroplasty is enhanced by attaining the correct balance between the intrinsic stability provided by plateau geometry and the presenting soft tissue structures. Patient selection, surgical proficiency and implant design are the three factors that shape clinical outcomes. The ORL-developed virtual version of the physical ASTM F1223-14 testing standard using ADAMS is able to discern differences in performance between TKA designs, is faster and less expensive than physical methods, solves the contemporary problem of obtaining predicate designs for comparison and has been accepted by the US Food and Drug Administration (US FDA) in a 510[k] submission.