Wear Testing
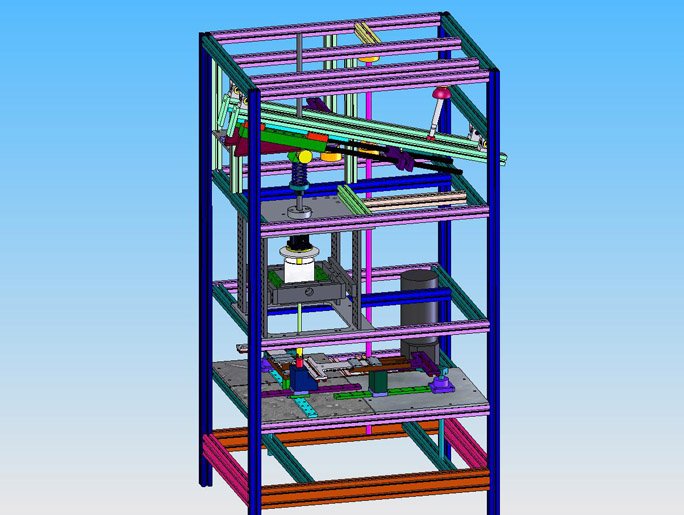
In 2004 through 2006, the PCM cervical prosthesis underwent 10 million cycles of testing per a customized FDA protocol and was subsequently cleared for clinical use in United States. Since that time, articulating and elastomeric lumbar and cervical disc replacement systems have been evaluated in the ORL proprietary, electro-mechanical joint simulators to satisfy FDA pre-clinical assessment requirements for the determination of safety and effectiveness.
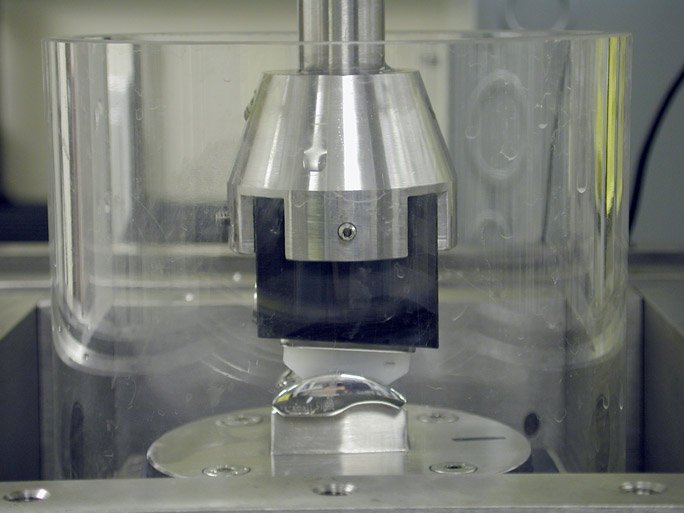
In 2005 through 2006, the STAR underwent 10 million cycles of testing per a customized FDA protocol and was subsequently cleared for clinical use in the United States.