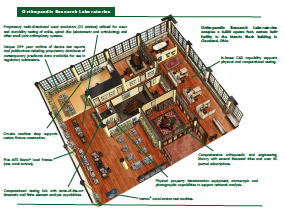
For more than four decades, ORL is recognized as an innovator of testing standards and methods with an extensive track record of providing information necessary for successful regulatory approvals, product quality confirmation, marketing materials and surgeon education. This Publications Library encapsulates presentation handouts that exemplify this expertise.
General
Musculoskeletal Allograft Tissue Safety
Mar 8, 2010 | General
The use of musculoskeletal allograft tissue in reconstructive orthopaedic procedures has markedly increased over the last decade. Surgeon knowledge of tissue bank practices in donor consent and...
The Evolving Role of Bone-Graft Substitutes
Mar 8, 2010 | General
It is estimated that more than 500,000 bone-grafting procedures are performed annually in the United States, with approximately half of these procedures related to spine fusion. These numbers easily...
Adverse Event Reporting – An FDA Requirement: What, When, Who & How
Mar 8, 2010 | General
Most orthopaedic surgeons in the course of their practice will encounter and explant medical devices which have failed. Such failures may simply result from long-term in-vivo device usage, component...
Orthopaedic Infection Prevention and Control: An Emerging New Paradigm
Mar 8, 2009 | General
More than 65 million inpatient and outpatient surgeries are done each year at U.S. hospitals. The Centers for Disease Control and Prevention (CDC) estimate that the rates of surgical site infection...
Bone Graft Substitutes: Facts, Fictions & Applications
Mar 8, 2009 | General
It is estimated that more than 500,000 bone-grafting procedures are performed annually in the United States, with approximately half of these procedures related to spine fusion. These numbers easily...
Adverse Event Reporting – An FDA Requirement: What, When, Who & How
Mar 8, 2009 | General
Most orthopaedic surgeons in the course of their practice will encounter and explant medical devices which have failed. Such failures may simply result from long-term in-vivo device usage, component...
Orthopaedic Infection: Community-Associated and Healthcare-Associated MRSA
Mar 8, 2008 | General
Methicillin-resistant Staphylococcus aureus (MRSA) is a strain of the staph infection resistant to beta-lactam antibiotics (penicillin, oxacillin, amoxicillin, and methicillin). MRSA is a bacterium...
Musculoskeletal Allograft Tissue Safety
Mar 8, 2008 | General
The use of musculoskeletal allograft tissue in reconstructive orthopaedic procedures has markedly increased over the last decade, Surgeon knowledge of tissue bank practices in donor consent and...
Bone Graft Substitutes: Facts, Fictions & Applications
Mar 8, 2008 | General
It is estimated that more than 500,000 bone-grafting procedures are performed annually in the United States, with approximately half of these procedures related to spine fusion. These numbers easily...
Adverse Event Reporting – An FDA Requirement: What, When, Who & How
Mar 8, 2008 | General
Most orthopaedic surgeons in the course of their practice will encounter and explant medical devices which have failed. Such failures may simply result from long-term in-vivo device usage, component...
Musculoskeletal Allograft Tissue Safety
Mar 8, 2007 | General
The use of musculoskeletal allograft tissue in reconstructive orthopaedic procedures has markedly increased over the last decade, Surgeon knowledge of tissue bank practices in donor gifting and...
Bone Graft Substitutes: Facts, Fictions & Applications
Mar 8, 2007 | General
It is estimated that more than 500,000 bone-grafting procedures are performed annually in the United States, with approximately half of these procedures related to spine fusion. These numbers easily...
The Influence of Antibiotics on the Fatigue Life of Acrylic Bone Cement: Assuring Clinical Structural Integrity
Mar 7, 2006 | General
Aseptic loosening attributed to cement fracture and the subsequent disruption of fixation interfaces remains a major long-term failure mode of cemented arthroplasty. Knowledge of the fracture...
Bone Graft Substitutes: Facts, Fictions & Applications
Mar 7, 2006 | General
It is estimated that more than 500,000 bone-grafting procedures are performed annually in the United States, with approximately half of these procedures related to spine fusion. These numbers easily...
Musculoskeletal Allograft Tissue Safety
Mar 7, 2006 | General
The use of musculoskeletal allograft tissue in reconstructive orthopaedic procedures has markedly increased over the last decade. Surgeon knowledge of tissue bank practices in donor consent and...
Highly Crosslinked Polyethylenes: Hopes vs. Realities
Mar 7, 2005 | General
The enduring success of the low-friction arthroplasty first advanced by Sir John Charnley as a solution for severe hip arthritic problems may be appreciated from the fact that in 2003 more than...
Antibiotic-Loaded Bone Cement in Aseptic Total Joint Replacement: Whys, Wherefores & Caveats
Mar 7, 2005 | General
Deep wound infection following joint replacement is one of the most devastating complications facing both the physician and patient. The use of antibiotic-loaded bone cement (ALBC) is a...
The Influence of Antibiotics on the Fatigue Life of Acrylic Bone Cement: Assuring Clinical Structural Integrity – Series II
Mar 7, 2005 | General
Aseptic loosening attributed to cement fracture and the subsequent disruption of fixation interfaces remains a major long-term failure mode of cemented arthroplasty. Knowledge of the fracture...
Musculoskeletal Allograft Tissue Safety
Mar 7, 2004 | General
The use of musculoskeletal allograft tissue in reconstructive orthopaedic procedures has markedly increased over the last decade, Surgeon knowledge of tissue bank practices in donor gifting and...
Antibiotic-Loaded Bone Cement in Aseptic Total Joint Replacement: Whys, Wherefores & Caveats
Mar 7, 2004 | General
Deep wound infection following joint replacement is one of the most devastating complications facing both the physician and patient. The use of antibiotic-loaded bone cement (ALBC) is a...
The Influence of Antibiotics on the Fatigue Life of Acrylic Bone Cement: Assuring Clinical Structural Integrity
Mar 7, 2004 | General
Aseptic loosening attributed to cement fracture and the subsequent disruption of fixation interfaces remains a major long-term failure mode of cemented arthroplasty. Knowledge of the fracture...
New Polys for Old: Contribution or Caveat?
Mar 7, 2003 | General
The enduring success of the low-friction arthroplasty first advanced by Sir John Charnley as a solution for severe hip arthritic problems may be appreciated from the fact that in 2001 more than...
Musculoskeletal Allograft Tissue Safety
Mar 7, 2003 | General
The use of Musculoskeletal allograft tissue in reconstructive orthopaedic procedures has markedly increased over the last decade, Surgeon knowledge of tissue bank practices in donor gifting and...
Bone Graft Substitutes: Facts, Fictions & Applications
Mar 7, 2003 | General
It is estimated that more than 500,000 bone-grafting procedures are performed annually in the United States, with approximately half of these procedures related to spine fusion. These numbers easily...
Antibiotic-Loaded Bone Cement in Aseptic Total Joint Replacement: Whys, Wherefores & Caveats
Mar 7, 2003 | General
Deep wound infection following joint replacement is one of the most devastating complications facing both the physician and patient. The use of antibiotic-loaded bone cement (ALBC) is a...
Assuring Cement Fixation: All Mixing Systems are NOT the Same
Mar 7, 2003 | General
Aseptic loosening attributed to cement fracture and the subsequent disruption of fixation interfaces remains a major long-term failure mode of cemented arthroplasty. Knowledge of the fracture...
New Polys for Old: Contribution or Caveat?
Mar 3, 2002 | General
The enduring success of the low-friction arthroplasty first advanced by Sir John Charnley as a solution for severe hip arthritic problems may be appreciated from the fact that in 2000 more than...
Musculoskeletal Allograft Tissue Safety
Mar 3, 2002 | General
The use of Musculoskeletal allograft tissue in reconstructive orthopaedic procedures has markedly increased over the last decade, Surgeon knowledge of tissue bank practices in donor gifting and...
Alternative Bearing Surfaces: The Good, Bad & Ugly
Mar 3, 2002 | General
The enduring success of the low-friction arthroplasty advanced by Sir John 2000, more than 270,000 hip arthroplasties were performed in the United States. Over the last three decades, patient...
Bone Graft Substitutes: Facts, Fictions & Applications
Mar 3, 2002 | General
It is estimated that more than 500,000 bone-grafting procedures are performed annually in the United States, with approximately half of these procedures related to spine fusion. These numbers easily...
Bone Graft Substitutes: Facts, Fictions & Applications
Mar 3, 2001 | General
Estimates of over 500,000 bone grafting procedures are performed annually in the United States, with approximately half of these procedures related to spine fusion. These numbers easily double on a...
Alternative Bearing Surfaces: The Good, Bad & Ugly
Mar 3, 2001 | General
The enduring success of the low friction arthroplasty advanced by Sir John Charnley as a solution for hip problems may be appreciated by the fact that in 1999, over 270,000 hip arthroplasties were...
New Polys for Old: Contribution or Caveat?
Mar 3, 2001 | General
The enduring success of the low friction arthroplasty first advanced by Sir John Charnley as a solution for severe hip arthritic problems may be appreciated from the fact that in 1999 over 500,000...